
1.25%
PILOCARPINE
For US Healthcare Professionals Only
16
INVESTIGATIONAL
FORMULATIONS
Initially evaluated in two
Phase 2b trials2
>1,000
PARTICIPANTS
Tested across Phase 2 studies 009 (N=157) and 010 (N=147), and Phase 3 trials GEMINI 1 (N=323) and GEMINI 2 (N=427)1,2
Mesopic +
photopic
CONDITIONS
Evaluated in both low light (mesopic) and bright light (photopic) conditions in Phase 3 studies3

1.25%
PILOCARPINE
Optimized concentration for presbyopia1,2

A proprietary vehicle that rapidly adjusts pH and increases corneal penetration*4-6
*Based on an in vitro study. Clinical significance is unknown.


The pH range of VUITY® in the bottle is between 3.5 and 5.5.1

Within seconds of contacting the ocular surface, VUITY® starts to adjust to the physiological pH (~pH 6.5) of the tear film.‡§,2,4
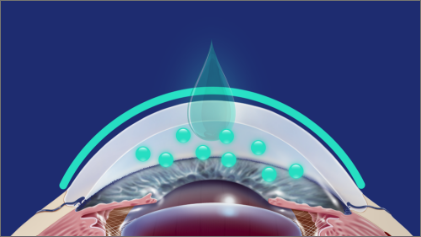
A neutral pH was associated with increased corneal penetration, improving ocular bioavailability.‡,4-6
‡Based on an in vitro study. Clinical significance is unknown.
§The physiological pH of tears (~pH 6.5) was achieved within 60 seconds.4
Pilocarpine hydrochloride is a cholinergic muscarinic agonist that activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ciliary muscle.1
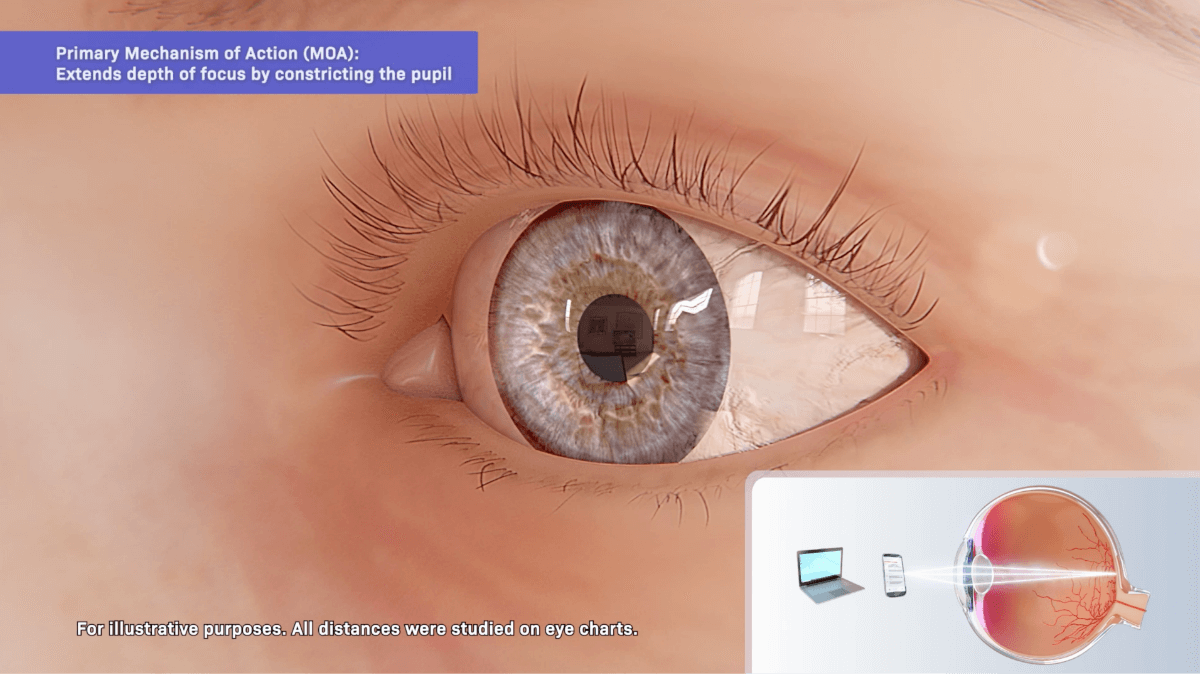
Pilocarpine hydrochloride is a cholinergic muscarinic agonist that activates muscarinic receptors located at smooth muscles such as the iris sphincter muscle and ciliary muscle.1
Primary:
Extends the depth of focus
VUITY® contracts the iris sphincter muscle, constricting the pupil to improve near and intermediate visual acuity while maintaining some pupillary response to light.
Secondary:
Increases the amplitude of accommodation¶
VUITY® also contracts the ciliary muscle and may shift the eye to a more myopic state.
¶Accommodation is the ability of the eye to change the refractive power of the lens to automatically focus on objects at various distances. Amplitude of accommodation (AA) is the amount of accommodation exerted to move the focus from the far point to the near point.11
VUITY® (pilocarpine hydrochloride ophthalmic solution) 1.25% is indicated for the treatment of presbyopia in adults.
VUITY is contraindicated in patients with known hypersensitivity to any ingredient in the formulation.
Miotics, including VUITY, may cause accommodative spasm. Patients should be advised not to drive or operate machinery if vision is not clear (e.g., blurred vision). In addition, patients may experience temporary dim or dark vision with miotics, including VUITY. Patients should be advised to exercise caution in night driving and other hazardous activities in poor illumination.
Rare cases of retinal detachment and retinal tear have been reported with miotics, including VUITY. Individuals with pre-existing retinal disease are at increased risk. Therefore, examination of the retina is advised in all patients prior to the initiation of therapy. Patients should be advised to seek immediate medical care with sudden onset of flashing lights, floaters, or vision loss.
VUITY is not recommended to be used when iritis is present because adhesions (synechiae) may form between the iris and lens.
Contact lens wearers should be advised to remove their lenses prior to the instillation of VUITY and to wait 10 minutes after dosing before reinserting their contact lenses.
To prevent eye injury or contamination, care should be taken to avoid touching the dispensing bottle to the eye or to any other surface.
The most common adverse reactions (>5%) reported in clinical trials were headache, conjunctival hyperemia, and eye irritation.
Please see full Prescribing Information.
US-VUI-230052
References: 1. VUITY [package insert]. North Chicago, IL: AbbVie Inc. 2.Price FW, et al. Ophthalmol Sci. 2021;1(4):100065. doi:https://doi.org/10.1016/j.xops.2021.100065. 3. Data on File, ABVRRTI73149. 4. Data on File, ABVRRTI73125. 5. Anderson RA, Cowle JB. Br Ophthalmol Sci.1968;52:607-611. 6. Irimia T, et al. Polymers. 2018;10(11):1221. doi:10.3390/polym10111221. 7. Suhonen P, et al. Eur Pharmaceutical.1998;6:169-176. 8. Abelson MB, et al. Arch Ophthalmol.1981;99(2):301. 9. Wolffsohn JS, Davies LN. Prog Retin Eye Res. 2019;68:124-143. 10. Data on File, ABVRRTI73127. 11. Abraham LM, et al. Indian J Ophthalmol Sci. 2005;53(2):105-108.